In the 4G era, the demand for arsenide PA is strong, and the global supply is in short supply: on the one hand, CMOS PA made of Si does not meet the trend of 3G / 4G high-speed rate and wide bandwidth due to the limitation of physical properties. It is expected that in the future, it will only share the market share of arsenide PA in the field of 2G low-end mobile phones, and 4G mobile phones will be the world of arsenide Pa; On the other hand, since 2G and 3G technologies adopt 4 to 5 different frequency bands respectively, and 4G mobile phones need to support nearly 40 network frequency bands, with the increase of the number of frequency bands supported by terminals, the demand for PA of arsenifiers will also increase from 1-2 in the 3G era to 4-5. The popularity of 4G mobile phones and the increase of the number of PA of single mobile phone will jointly create a strong demand for arsenifiers all over the world, Arsenic chemists continue to be in short supply.
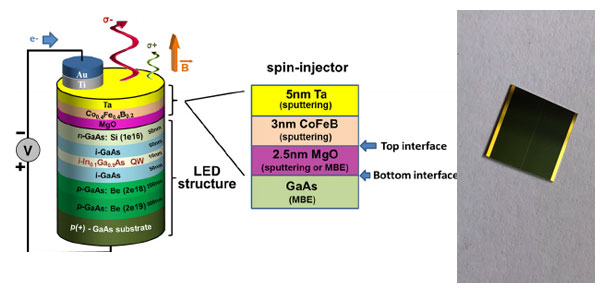
GaAs, GaAsP, or GaP construct LED contact device. The junction during a LED is forward biased and when electrons cross the junction from the n- to the p-type material, the electron-hole recombination process produces some photons within the IR or visible during a process called electroluminescence.
When the applied forward voltage on the diode of the LED drives the electrons and holes into the active region between the n-type and p-type material, the energy are often converted into infrared or visible photons. this suggests that the electron-hole pair drops into a more stable bound state, releasing energy on the order of electron volts by emission of a photon. The red extreme of the color spectrum , 700 nm, requires an energy release of 1.77 eV to supply the quantum energy of the photon. At the opposite extreme, 400 nm within the violet, 3.1 eV is required.